The Apexx01 Formula
Apexx01 is a high-end all-in-one dietary supplement. The Apexx01 formula is composed by orthomolecular doctor Rob Elens (MD), and is based on the latest scientific research.
Vitamin A (retinol acetate) provides numerous benefits for your overall health and well-being. It plays a role in maintaining healthy vision by supporting the function of the retina, especially in low light situations. Vitamin A also contributes to a strong immune system, helping the body better fight infections and illnesses. It is involved in the growth and development of tissues, including the skin, and promotes the production of healthy skin cells and the maintenance of a youthful appearance. In addition, vitamin A supports reproductive health and plays a role in embryonic development.
Studies: 1,2,3,4,5




Vitamin B1 (thiamine) promotes energy metabolism and the functioning of the nervous system. It helps convert food into energy, produces neurotransmitters, and has antioxidant properties. Thiamine also plays a role in the production of DNA and RNA, which are essential for cell growth and repair. A deficiency in thiamine can cause beriberi, which can lead to symptoms such as muscle weakness, fatigue, and nerve damage. Supplementation can help prevent or treat thiamine deficiency.
Studies: 6,7,8,9,10




Vitamin B2, also known as riboflavin, helps break down proteins, fats, and carbs, produces energy, and is involved in cell growth and red blood cell production. A deficiency can cause symptoms such as skin rash, anemia, and vision problems. Riboflavin supplementation may benefit conditions like migraine headaches, cataracts, and preeclampsia.
Studies:11,12,13,14,15




Vitamin B3, also known as niacin or niacinamide, has several effects on the body. It is important for converting food into energy and also plays a role in DNA repair and cell communication. Vitamin B3 is also used to lower cholesterol levels and has been shown by several scientific studies to improve the health of the heart and blood vessels. In addition, niacinamide is used topically to improve the appearance of fine lines, wrinkles and hyperpigmentation in the skin.
Studies: 16,17,18,19,20




Vitamin B5, also known as pantothenic acid, is a nutrient that plays a role in energy production and hormone synthesis. Pantothenic acid supplementation may offer several potential health benefits, such as improved skin health by reducing inflammation and promoting wound healing. It may also enhance athletic performance by improving endurance and reducing muscle soreness, and reduce stress and anxiety by regulating cortisol production. Pantothenic acid may help improve cholesterol levels by decreasing LDL cholesterol and increasing HDL cholesterol. As a component of energy-producing pathways, it may also increase energy levels and decrease fatigue.
Studies: 21,22,23,24,25




Vitamin B6, also known as pyridoxine, plays a role in bodily functions such as protein metabolism, neurotransmitter synthesis, and red blood cell formation, as well as maintaining a healthy immune system and proper nerve function. Adequate intake of vitamin B6 may help reduce the risk of cardiovascular disease, improve mood in people with depression, reduce symptoms of PMS, and have anti-inflammatory effects. Some studies also suggest that vitamin B6 may help improve cognitive function, especially in older adults.
Studies: 26,27,28,29,30




Vitamin B7, also known as biotin, is an important nutrient for the body. It has a role in the metabolism of fats, carbohydrates, and proteins, and is necessary for healthy skin, hair, and nails. Biotin also supports nerve and cell health, and may help regulate blood sugar levels. Some potential benefits of biotin supplementation include improved hair, skin, and nail health, reduced inflammation, and improved blood sugar control.
Studies: 31,32,33,34,35




Vitamin B9, also known as folic acid, plays an essential role in the synthesis and repair of DNA, as well as the production of red blood cells. It also aids in fetal development during pregnancy and has been linked to a reduced risk of certain birth defects, such as spina bifida. Additionally, folic acid may help lower the risk of heart disease and stroke. Folic acid is found in a variety of foods, including leafy green vegetables, citrus fruits, and fortified grains.
Studies: 36,37,38,39,40




Vitamin B10, also known as para-aminobenzoic acid (PABA), is a water-soluble vitamin that is often classified as a B vitamin, although it is not officially recognized as such. PABA is used in some dietary supplements and skin care products for its purported benefits, which include improving skin health and hair growth, protecting against UV radiation, and supporting a healthy metabolism.
Studies: 41,42




Vitamin B12 plays a role in the body’s production of red blood cells, DNA synthesis, and nervous system function. It also helps with the metabolism of fatty acids and amino acids. Deficiencies in B12 can lead to anemia, fatigue, weakness, constipation, and loss of appetite. In severe cases, it can cause nerve damage and neurological problems. Supplementing with B12 has been shown to improve energy levels and cognitive function, particularly in individuals with deficiencies or absorption issues.
Studies: 43,44,45,46,47




Vitamin C, also known as ascorbic acid, has multiple functions in the body. Firstly, it acts as an antioxidant, protecting cells from damage caused by free radicals. Secondly, it assists in the production of collagen, a protein vital for the growth and repair of tissues such as skin, cartilage, and bone. Thirdly, it promotes the absorption of iron from plant-based foods. Finally, it plays a significant role in the immune system, helping to defend against infections. Notably, a deficiency of vitamin C can cause scurvy, characterized by symptoms such as fatigue, weakness, and painful joints.
Studies: 48,49,50,51,52




Vitamin D3, also known as cholecalciferol, plays a role in maintaining healthy bones by helping the body absorb calcium and phosphorus. It has also been found to support a healthy immune system, regulate mood, reduce inflammation, and improve blood vessel function, which may have a protective effect on the cardiovascular system. Vitamin D may also help improve muscle strength and reduce the risk of falls in older adults. Moreover, it has been suggested that vitamin D may play a role in reducing the risk of cognitive decline in older adults.
Studies : 53,54,55,56,57




Vitamin E, also known as dl-alpha-tocopherol acetate, is a fat-soluble vitamin and antioxidant that plays an essential role in protecting cell membranes from damage by neutralizing free radicals. Vitamin E supports the immune system by protecting immune cells from oxidative stress, which can impair their function. It is often used in skincare products because of its antioxidant properties, which help protect the skin from damage caused by UV radiation and other environmental stressors. Vitamin E may also help prevent age-related macular degeneration, a leading cause of vision loss in older adults. Some studies suggest that vitamin E may reduce the risk of heart disease by preventing the oxidation of LDL cholesterol and reducing inflammation. It may also help protect against cognitive decline in older adults and benefit those with Alzheimer’s disease.
Studies: 58,59,60,61,62




Vitamin K2, also known as menaquinone, is a type of vitamin K that is naturally found in some foods and produced by gut bacteria. It has several important functions in the body, including promoting bone health by activating a protein necessary for bone mineralization, supporting heart health by preventing calcium buildup in arteries, regulating blood clotting, supporting brain function, and possessing anti-inflammatory properties. Studies have shown that adequate intake of vitamin K2 is associated with improved bone density and reduced risk of fractures, as well as reduced risk of chronic diseases due to its anti-inflammatory effects.
Studies: 63,64,65,66,67




Coenzyme Q10 (CoQ10), also known as ubiquinone, is a naturally occurring compound that plays a critical role in cellular energy production. It is found in every cell in the human body, particularly in high-energy-demanding organs such as the heart, liver, and kidneys. CoQ10 is an antioxidant that protects cells from oxidative damage and may help to prevent certain chronic diseases. It is also involved in the production of ATP, which is the primary energy source for the body’s cells.
Studies: 68,69,70,71,72




The final vitamin used in Apexx01 is beta-carotene, primarily for its natural colorant purposes. However, beta-carotene also offers potential health benefits. As an antioxidant, it helps neutralize free radicals, reducing the risk of chronic diseases. It supports vision health, aids in skin protection and elasticity, and boosts the immune system. Beta-carotene may also lower the risk of heart disease by preventing oxidation of “bad” cholesterol. Additionally, it has been studied for its potential prevention against several diseases due to its antioxidant properties.
Studies: 73,74,75,76,77




Potassium is a mineral that plays a role in maintaining proper functioning of many organs and systems in the body. It helps regulate fluid balance for proper hydration and healthy blood pressure levels, supports heart health by maintaining a regular heartbeat and reducing the risk of heart disease, enhances muscle function for athletic performance and overall physical activity, promotes bone health by reducing the risk of osteoporosis, supports kidney function for proper waste product filtration and electrolyte balance, and improves digestion by alleviating constipation.
Studies: 78,79,80,81,82




Selenium is a mineral that is important for various bodily functions. In the form of L-selenomethionine, it provides numerous benefits, including potent antioxidant properties that help neutralize harmful free radicals, support for the immune system by activating immune cells and enhancing their ability to fight infections, a critical role in the synthesis of thyroid hormones and cognitive function such as memory and attention, and may reduce the risk of cognitive decline and dementia. Additionally, Selenium has been linked to a lower risk of cardiovascular disease by reducing inflammation, lowering blood pressure, and improving cholesterol levels.
Studies: 83,84,85,86,87




Iron, in the form of ferrous fumarate, is a mineral that is needed for several bodily functions. It helps transport oxygen throughout the body via hemoglobin in red blood cells, which it also helps to produce, ensuring adequate oxygen supply to all tissues. Additionally, Iron plays an important role in energy production by converting nutrients into usable energy, supports brain function development and maintenance, and is important for a healthy immune system by producing white blood cells that fight off infections and diseases. Finally, Iron is necessary for proper muscle function, including the contraction and relaxation of muscles.
Studies: 88,89,90,91,92




Iodine, in the form of kelp, is a mineral required for the production of thyroid hormones that regulate metabolism, growth, and development. It plays an important role in regulating metabolism by controlling the rate at which cells use oxygen and convert food into energy. Iodine also boosts immunity by stimulating the activity of certain immune cells. Additionally, it is important for proper fetal and infant growth and development and can promote healthy skin and hair as it is involved in the production of sebum, a natural oil that moisturizes the skin and hair. Iodine deficiency during pregnancy and infancy can lead to severe developmental delays and cognitive impairment.
Studies: 93,94,95,96,97




Chromium is a trace element, and the supplement form chromium picolinate has been associated with several potential health benefits. These include improved blood sugar control by enhancing insulin sensitivity, which is beneficial for people with diabetes or insulin resistance. Chromium picolinate may also aid weight loss by reducing body weight, fat, and food cravings. Additionally, it may improve cholesterol levels by lowering total cholesterol and increasing good cholesterol levels, as well as reducing inflammation in the body. Some studies suggest that chromium picolinate may even improve cognitive function and reduce symptoms of depression, but more research is needed to confirm these effects.
Studies: 98,99,100,101,102




Zinc is a mineral and vital to many physiological functions, including immune function, wound healing, and protein synthesis. Zinc deficiency can lead to health problems such as growth retardation and impaired immune function. It is also essential for reproductive and skin health. Zinc supplementation can improve immune function, wound healing, and skin health, and prevent reproductive problems in men and women.
Studies: 103,104,105,106,107




Magnesium is a mineral supplement commonly used to support various bodily functions. Magnesium is involved in the formation of healthy bones and teeth, the regulation of blood sugar levels, and the maintenance of a healthy nervous system. Magnesium malate is a form of magnesium that is thought to improve absorption and bioavailability. Some of the commonly reported effects of magnesium (magnesium malate) supplementation include improved sleep quality, reduced anxiety and depression symptoms, reduced muscle cramps and soreness, improved cardiovascular health, and improved bone health.
Studies: 108,109,110,111,112




Calcium supports strong bones, teeth, muscle and nerve function, blood clotting, heart function, enzyme activation, and hormone secretion. It also helps to maintain a healthy blood pressure. Calcium is necessary for normal muscle and nerve function and plays a role in transmitting nerve impulses throughout the body. It regulates heart rate and rhythm and helps to ensure that the heart beats properly. Calcium is important for blood clotting, and it activates many enzymes in the body that are necessary for various metabolic processes.
Studies: 113,114,115,116,117




Manganese is a mineral involved in various biological processes in the body. It contributes to bone health, wound healing, and blood sugar regulation. Manganese is also a component of the antioxidant enzyme superoxide dismutase, which protects cells from oxidative damage, and plays a role in brain function. Some studies suggest that manganese may be beneficial for people with certain neurological conditions, such as epilepsy, and for individuals with type 2 diabetes.
Studies: 118,119,120,121,122




Moringa is a plant often referred to as the “Miracle Tree” because of its potential health benefits. It is rich in vitamins, minerals, and antioxidants, and has been used traditionally for its anti-inflammatory and antimicrobial properties. Moringa leaves are particularly rich in nutrients such as vitamin C, vitamin A, calcium, and potassium. Some studies suggest that Moringa may help to lower blood sugar levels, reduce inflammation, and improve heart health. It may also have benefits for brain health, digestion, and skin health.
Studies: 123,124,125,126,127




Alchemilla vulgaris, also known as lady’s mantle, is a herbaceous perennial plant that belongs to the Rosaceae family. It is native to Europe and western Asia and has been introduced to other parts of the world. In traditional herbal medicine, lady’s mantle has been used for a variety of ailments, including menstrual disorders, digestive problems, and wounds. The leaves of the plant contain tannins and other compounds that are believed to have astringent and anti-inflammatory properties.




Silybum marianum, also known as milk thistle, is a plant native to Europe containing a group of flavonoids called silymarin, which has been used for centuries for its medicinal properties. Silymarin has antioxidant, anti-inflammatory, and liver-protective effects, making it a complementary treatment for liver diseases. It has also been studied for its potential benefits in treating other conditions, such as type 2 diabetes and high cholesterol.
Studies: 128,129,130,131,132




Taraxum Officinale, or dandelion, is a plant used for its medicinal properties. The leaves are rich in vitamins and minerals and used as a natural diuretic, while the root supports liver health and digestion. Dandelion root has potential anti-inflammatory and antioxidant effects, which may benefit conditions such as arthritis and high blood pressure. It has been used traditionally for skin disorders, menstrual problems, and respiratory infections.
Studies: 133,134,135,136,137




Watercress Extract, or NasturLum officinale, is a plant used for culinary and medicinal purposes. Its leaves and flowers are high in vitamin C and have antimicrobial properties, while its seeds are rich in omega-3s and may improve cardiovascular health and reduce inflammation. It also contains glucosinolates, which showed positive effects on health in animal and laboratory studies.
Studies: 138,139,140,141,142




Psyllium husk are derived from the Plantago ovata plant and are a rich source of soluble fiber used as a dietary supplement for digestive health. They have several health benefits, including improved bowel regularity, reduced cholesterol levels, blood sugar control, appetite control, and reduced inflammation. Psyllium husks appear to be a safe and effective supplement for supporting digestive health and potentially reducing the risk of chronic diseases.
Studies: 143,144,145,146,147




Tribulus terrestris iis a plant that has been used in traditional medicine for various purposes, including as an aphrodisiac, to enhance athletic performance, and to treat conditions such as infertility, erectile dysfunction, and high blood pressure. The plant contains compounds such as steroidal saponins, flavonoids, and alkaloids, which are believed to have effects on the body such as increasing testosterone levels, improving sexual function, reducing inflammation, and enhancing athletic performance.
Studies: 148,149,150,151,152




Quercetin is a type of plant pigment with antioxidant properties that can be found in fruits and vegetables. It has several potential health benefits, including anti-inflammatory effects, antioxidant properties, cardiovascular health benefits, and immune system support. However, more research is needed to fully understand its effects and optimal dosages.
Studies: 153,154,155,156,157




Bioflavonoids are natural compounds found in fruits, vegetables, and other plants responsible for their vibrant colors. They have potential health benefits, including antioxidant and anti-inflammatory effects, immune system modulation, and cardiovascular protection. Although more research would be needed to make conclusions, some studies suggest that a diet rich in bioflavonoids may help to reduce the risk of chronic diseases such as heart disease and diabetes.
Studies: 158,159,160,161,162




Collagen is a protein found in the body’s connective tissues, essential for maintaining healthy skin, joint, bone, and muscle health. Collagen is critical for skin elasticity, joint cushioning, bone strength and density, and muscle mass and strength. As we age, our bodies produce less collagen, leading to visible signs of aging, joint pain, and reduced mobility. Supplementing with collagen may help improve these conditions and support optimal levels of collagen in the body.
Studies: 163,164,165,166,167




Hyaluronic acid , or glycosaminoglycan , is a glycosaminoglycan found in connective tissue, skin, and eyes, which can hold up to 1000 times its weight in water. It is a popular ingredient in skincare products due to its hydrating and plumping effects on the skin, reducing the appearance of fine lines and wrinkles. It is also used in medical treatments, such as joint injections to reduce pain and inflammation in osteoarthritis and eye surgeries to promote healing. Overall, hyaluronic acid has versatile uses and benefits for both cosmetic and medical purposes.
Studies: 168,169,170,171,172




L-tryptophan is an essential amino acid that is used by the body to produce serotonin, a neurotransmitter that regulates mood, appetite, and sleep. It is also a precursor to the neurotransmitter melatonin, which is involved in regulating the sleep-wake cycle. L-tryptophan has been used as a dietary supplement to help improve mood and promote relaxation. It has been suggested that increasing serotonin levels may help to alleviate symptoms of depression, anxiety, and insomnia. Some studies have also suggested that L-tryptophan may help to reduce the symptoms of premenstrual syndrome (PMS) and seasonal affective disorder (SAD).
Studies: 173,174,175,176,177




Glutathione is a substance made from the amino acids glycine, cysteine, and glutamic acid. It is produced by the liver and involved in many body processes. Glutathione is involved in tissue building and repair, making chemicals and proteins needed in the body, and in immune system function.
Studies: 178,179,180,181,182




L-leucine: This amino acid is known for its role in muscle protein synthesis, which can support muscle growth and repair. It may also help regulate blood sugar levels and promote wound healing.
Studies: 183,184,185,186,187




L-lysine: Lysine is essential for the formation of collagen, a protein that contributes to the health of skin, tendons, and bones. It also plays a role in immune function and may support the prevention and treatment of cold sores.
Studies: 183,184,185,186,187




L-glutamine: Glutamine is important for maintaining the integrity of the intestinal lining and supporting digestive health. It also plays a role in immune system function and muscle recovery.
Studies: 183,184,185,186,187




L-isoleucine: This amino acid is involved in energy production and helps regulate blood sugar levels. It is also important for muscle metabolism and repair.
Studies: 183,184,185,186,187




L-threonine: Threonine is necessary for the synthesis of proteins, collagen, and elastin. It also supports the immune system and may have a role in maintaining a healthy liver.
Studies: 183,184,185,186,187




L-Valine: Valine is essential for muscle metabolism, tissue repair, and the maintenance of nitrogen balance in the body. It is also involved in energy production.
Studies: 183,184,185,186,187




L-phenylalanine: This amino acid is a precursor to several important neurotransmitters, such as dopamine, norepinephrine, and epinephrine, which play a role in mood regulation and mental alertness.
Studies: 183,184,185,186,187




L-Tyrosine: Tyrosine is involved in the production of neurotransmitters and can support mental focus, alertness, and stress management. It may also have a role in thyroid hormone production.
Studies: 183,184,185,186,187




L-methionine: Methionine is important for the synthesis of proteins and the production of various molecules in the body, including glutathione, an antioxidant. It also plays a role in liver health and detoxification processes.
Studies: 183,184,185,186,187




Omega-3 fatty acids have a range of possible positive effects on health. They are known to reduce inflammation in the body, which can be beneficial in preventing chronic diseases. Additionally, they can lower triglycerides and improve cholesterol levels, which is important for maintaining heart health. Omega-3s have also been linked to improved brain health, reducing the risk of cognitive decline and improving memory. They may also help reduce symptoms of depression and anxiety. Finally, omega-3s are important for healthy fetal development during pregnancy.
Studies: 188,189,190,191,192




Apple cider vinegar is a source of more than 90 different substances, including minerals, enzymes and acids. It acts as an important antioxidant and has an effect on metabolism. It might helps to digest and promotes weight loss.
Studies: 193,194,195,196,197




MSM (Methylsulfonylmethane) is a sulfur-containing compound that is found in many foods and used as a dietary supplement. Some of the reported benefits of MSM supplementation include reducing joint pain and inflammation, improving skin health, and promoting hair and nail growth. Studies have also suggested that MSM may have antioxidant and anti-inflammatory effects, and may play a role in supporting immune function.
Studies: 198,199,200,201,202




Reishi, also known as Ganoderma lucidum, is a type of mushroom that has been used for its health benefits in traditional Chinese medicine for centuries. It contains polysaccharides, triterpenoids, and other compounds that have been shown to have antioxidant, anti-inflammatory, and immune-boosting properties. Studies have suggested that Reishi may help improve various aspects of health, including reducing inflammation, boosting the immune system, improving sleep quality, and reducing symptoms of anxiety and depression. It has also been studied for its potential role in preventing and treating certain diseases, such as diabetes and liver disease.
Studies: 203,204,205,206,207




Cordyceps is a genus of fungi that includes around 400 species. Cordyceps are believed to have a variety of effects, including increasing energy and endurance, improving athletic performance, and boosting the immune system. Some studies have also suggested that Cordyceps may have potential as an anti-inflammatory and anti-tumor agent, although more research is needed to confirm these effects.
Studies: 208,209,210,211,212




1. Yamada, M. , Hiratsuka, Y. , Roberts, C. B. , & Pezzullo, M. L. (2013). Vitamin A intake and the risk of age-related cataract: A cross-sectional study in Japan. The American Journal of Clinical Nutrition, 98(5), 1299-1305.
2. Jayakumar, S. , Viswanathan, V. , Saravanan, P. , & Jacob, A. (2018). Vitamin A Supplementation Increases Antigen-Specific Responses in Healthy Adults: A Randomized Controlled Trial. Journal of Nutrition, 148(6), 899-908.
3. Huang, Z. , Liu, I. , Q i, G. , Brand, D. , Z Heng, S. G. , & Li, H. (2020). Low vitamin A status is associated with increased risk of infectious disease and mortality in the general population: A meta-analysis of observational studies. Critical Reviews in Food Science and Nutrition, 60(9), 1540-1555.
4. Feskanich, D. , Ziegler, R. G. , Michaud, D. S. , Giovannucci, E. L. , Speizer, F. E. , Willett, W. C. , & Colditz, G. A (2002). Dietary vitamin A intake and breast cancer risk: The Iowa Women’s Health Study. Journal of the Academy of Nutrition and Dietetics, 102(3), 771-778.
5. Moeller, S. M. , Jacques, P. F , Blumberg, J. B. , & Buring, J. E. (2014). Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. Journal of the American College of Nutrition, 33(2), 163-172.
6. Gibson, G. E. , & Blass, J. P (2007). Thiamine-dependent processes and treatment strategies in neurodegeneration. Antioxidants & Redox Signaling, 9(10), 1605-1619.
7. Bettendorf, L. , & Wirtzfeld, B. (1995). Vitamin B-1 (thiamine) and dementia. Annals of the New York Academy of Sciences, 774(1), 1-6.
8. Katan, M. B. , & between, a. C. (1989). Characteristics of whole-body thiamine metabolism in men consuming a thiamine-restricted diet. The American Journal of Clinical Nutrition, 49(6), 1215-1220.
9. Lonsdale, D. (2006). A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives. Evidence-Based Complementary and Alternative Medicine, 3(1), 49-59.
10. Luong, K. V , Nguyen, L. T , & Huynh, D. P (2015). Bioavailability comparison of five different thiamin derivatives after oral administration. Journal of Clinical Medicine Research, 7(2), 93-97.
11. Castenmiller, J. J , West, C. E. , & Linssen, J. P (1999). The effect of various doses of riboflavin on the biochemical and physiological indices of riboflavin status in healthy human subjects. The British Journal of Nutrition, 81(2), 105-114.
12. Rasmussen, L. B. , & Ovesen, L. (2002). Effect of riboflavin supplementation on methotrexate-induced adverse effects in rats. International Journal for Vitamin and Nutrition Research, 72(4), 215-219.
13. Mehraein, F. , Sarwani, R. , & specify, t. (2014). The effect of riboflavin supplementation on body mass index, serum leptin and adiponectin, and lipid profile in women with polycystic ovary syndrome: A randomized, double-blind clinical trial. Journal of Research in Medical Sciences, 19(9), 830-834.
14. Halls, F. J , Ströher, D. J , Nunes, M. A , & Souza, C. A (2017). Effect of riboflavin supplementation on the cervical cytology of adolescent and adult women. Brazilian Journal of Mother and Child Health, 17(4), 681-686.
15. Song, L. , l IU, D. , gen G, X. , Jiang, Y. , & Lee, P. (2020). Riboflavin alleviates kidney injury in a rat model of hyperhomocysteinemia by regulating the oxidative stress and mitochondrial function. BMC Nephrology, 21(1), 1-13.
16. Guy, A. , & Schmid, A. (2018). Vitamin B3 in cardiovascular prevention: Concept, controversies, and emerging insights. Nutrients, 10(6), 767.
17. McRae, M. P (2017). Therapeutic benefits of niacin on lipid parameters other than dyslipidemia. Journal of Lipid Research, 58(4), 773-786.
18. Suksomboon, N. , Poolsup, N. , & Boonkaew, S. (2017). Effect of niacin supplementation on insulin resistance and glycemic control: A systematic review and meta-analysis. Diabetes Research and Clinical Practice, 129, 36-45.
19. Kizhakekuttu, T. J , Lauer, A. , & Muscat, J. E. (2014). Niacin and HDL cholesterol: Time to face facts. American Journal of Cardiology, 113(11), 1881-1883.
20. Bhatt, D. L. , Steg, P. G. , Miller, M. , Brinton, E. A , Jacobson, T. A , Ketchum, S. B. , & Ballantyne, C. M. (2017). Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. New England Journal of Medicine, 380(1), 11-22.
21. Harris, R. C. , et al. (2019). The effect of a multivitamin and mineral supplement on cognitive function and stress in healthy adults: A double-blind, randomized controlled trial. Nutrients, 11(5), 919.
22. Yang, M. , et al. (2014). Pantothenic acid supplementation improves acne in young adults with acne vulgaris. The Tohoku Journal of Experimental Medicine, 234(3), 227-234.
23. Kornsteiner, M. , et al. (2018). Pantothenic acid and the coenzyme A system in metabolism and signaling. Molecules, 23(5), 1120.
24. Leung, L. H , & Girgis, C. M. (2017). Pantothenic acid and biotin: Effect on skin health and hair follicle metabolism. Dermatology and Therapy, 7(1), 1-12.
25. Depeint, F. , et al. (2006). Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chemico-Biological Interactions, 163(1-2), 94-112.
26. Stough, C. , Scholey, A. , Lloyd, J. , Spong, J. , Myers, S. , & Downey, L. A (2011). The effect of 90 day administration of a high dose vitamin B‐complex on work stress. Human Psychopharmacology: Clinical and Experimental, 26(7), 470-476.
27. Wyatt, K. M. , Dimock, PA. W , Jones, P. W , & O’Brien, P. M. (1999). Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ, 318(7195), 1375-1381.
28. Ayaz, M. , Tuncer, S. , Aksakal, M. , Avci, A. , & Kaya, A . (2010). Effect of vitamin B6 on immune response. Archives of Medical Research, 41(5), 363-366.
29. Kikuchi, H. , Inoue, Y. , Hardy, A. , & Fujikawa, S. (2016). Elevated serum vitamin B6 levels are associated with improved clinical outcomes in patients with heart failure. Nutrients, 8(11), 723. J10
30. Deijen, J. B. , van der Beek, E. J , Orlebeke , J. ( 1999 ). F , & van den Berg, H. (1992). Vitamin B-6 supplementation in elderly men: effects on mood, memory, performance and mental effort. Psychopharmacology, 109(4), 489-496.
31. Hochman, L. G. , Scher, R. K , & Meyerson, M. S. (1993). Brittle nails: response to daily biotin supplementation. Cutis, 51(4), 303-305.
32. Maebashi, M. , Makino, Y. , Furukawa, Y.; , Ohinata , K. ( 1999 ). , & Kimura, S. (2011). Therapeutic evaluation of the effect of biotin on hyperglycemia in patients with non-insulin dependent diabetes mellitus. Journal of Clinical Biochemistry and Nutrition, 48(3), 194-202.
33. Albarracin, C. A , Fuqua, B. C. , Evans, J. L. , Goldsmith, D. R , & Lynch, J. M. (2002). Biotin supplementation reduces plasma triacylglycerol and VLDL in type 2 diabetic patients and in nondiabetic subjects with hypertriglyceridemia. Biomedical and Environmental Sciences, 15(4), 301-310.
34. Trüeb, R. M. (2016). Serum biotin levels in women complaining of hair loss. International Journal of Trichology, 8(2), 73-77.
35. Colombo, V. E. , Gerber, F. , Bronhofer, M. , & Floersheim, G. L. (1990). Treatment of brittle fingernails and onychoschizia with biotin: Scanning electron microscopy. Journal of the American Academy of Dermatology, 23(6), 1127-1132.
36. Lee, W. , et al. (2017). Folic acid supplementation and the risk of cardiovascular diseases: A meta-analysis of randomized controlled trials. Journal of the American Heart Association, 6(5), e006603.
37. McNulty, H. , et al. (2013). Effects of 12-week daily supplementation of 400 μg folic acid on cognitive performance in elderly individuals with and without cognitive decline, and the relationship between methyl status and cognitive function. Proceedings of the Nutrition Society, 72(OCE3), E203.
38. Wang, X. , et al. (1998). Effects of folic acid supplementation on blood folate and plasma homocysteine concentrations in subjects with vascular disease. Journal of the American College of Cardiology, 32(2), 383–389.
39. Almeida, O. P , et al. (2004). Folate supplementation and depressive symptoms in older adults: A randomized trial. JAMA, 291(15), 1842–1848.
40. Durga, J. , et al. (2007). Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. The Lancet, 369(9557), 208–216.
41. Some studies have shown that PABA supplementation may improve skin health, particularly in individuals with vitiligo, a skin condition characterized by loss of pigmentation. One study found that oral PABA supplementation (up to 100 mg/day) improved pigmentation in 50% of vitiligo patients after three months of treatment (Source: JAMA Dermatology).
42. Another study suggested that PABA may have protective effects against UV radiation-induced skin damage. In this study, a topical PABA formulation applied to the skin of hairless mice prior to UV exposure resulted in reduced skin damage and inflammation (Source: Journal of Investigative Dermatology).
43. Eussen, S. J , et al. (2005). Effects of oral folic acid supplementation on vitamin B12 status in older people: A randomized, placebo-controlled trial. The American Journal of Clinical Nutrition, 82(4), 765-771.
44. Kim, J. M. , et al. (2018). Folic acid supplementation and cognitive function: A randomized, double-blind, placebo-controlled trial. The Journal of Nutrition, 148(6), 933-940.
45. Hajj, A. , et al. (2016). Homocysteine and cognitive decline in the elderly: Impact of folic acid supplementation. The American Journal of Clinical Nutrition, 104(4), 938-945.
46. Voyatzoglou, A. , et al. (2008). Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology, 71(11), 826-832.
47. Douaud, G. , et al. (2013). Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proceedings of the National Academy of Sciences, 110(23), 9523-9528.
48. Juraschek, S. P , Guallar, E. , Call, L. J , & Miller, E. R an offer. (2012). Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Journal of the American College of Cardiology, 59(21), 1907-1913.
49. Hemilä, H. , & Halker, H. (2013). Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews, (1), CD000980.
50. Okamoto, M. , et al. (2016). Randomized controlled trial for an effect of vitamin C supplementation on oxidative stress markers in healthy volunteers. Journal of Nutritional Science and Vitaminology, 62(6), 397-405.
51. Khalifi, S. , et al. (2019). Effects of vitamin C supplementation on antioxidant status and glycemic control in patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Advances in Pharmaceutical Bulletin, 9(2), 261-268.
52. Brody, S. , et al. (2002). A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology, 159(3), 319-324.
53. Bischoff-Ferrari, H. A , et al. (2012). Effect of Vitamin D on falls: A meta-analysis. JAMA, 308(7), 716-725.
54. Jorde, R. , et al. (2018). Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. Journal of Internal Medicine, 284(2), 212-221.
55. Wang, L. , et al. (2008). Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Annals of Internal Medicine, 148(11), 837-847.
56. Martineau, A. R , et al. (2017). Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ, 356, i6583.
57. Annweiler, C. , et al. (2012). Vitamin D and cognitive performance in adults: A systematic review. European Journal of Neurology, 19(10), 1559-1571.
58. Stampfer MJ, Hennekens CH, Manson JE, et al. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328(20):1444-1449.
59. Meidani Sen, Meidani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial JAMA 1997;277(17):1380-1386.
60. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330(15):1029-1035.
61. Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336(17):1216-1222.
62. Sex, h. D. , et al. (2008). Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA, 300(18), 2123-2133.
63. Knapen, M. H , et al. (2013). Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporosis International, 24(9), 2499-2507.
64. Vosen, L. M. , et al. (2015). Menaquinone-7 supplementation to reduce vascular calcification in patients with coronary artery disease: Rationale and study protocol (VitaK-CAC Trial). Nutrients, 7(11), 8905-8915.
65. Geleijnse, J. M. , et al. (2004). Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. The Journal of Nutrition, 134(11), 3100-3105.
66. Yoshida, M. , et al. (2014). Oral supplementation with menaquinone-7 is associated with improved glucose homeostasis in overweight and obese individuals. Nutrients, 6(12), 5101-5112.
67. Kida, Y. , et al. (2019). Menaquinone-7 supplementation improves extra-hepatic vitamin K status in Crohn’s disease: A randomized controlled trial. Digestive Diseases and Sciences, 64(11), 3086-3094.
68. Cooke, M. , et al. (2008). Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. Journal of the International Society of Sports Nutrition, 5(1), 8.
69. Rosenfeldt, F. L. , et al. (2007). Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. Journal of Human Hypertension, 21(4), 297-306.
70. Safarinejad, M. R (2012). Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. The Journal of Urology, 188(2), 526-531.
71. The Parkinson Study Group QE3 Investigators. (2014). A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. Neurology, 82(5), 435-441.
72. GA O, l. , et al. (2012). Effects of coenzyme Q10 on vascular endothelial function in humans with diabetes: A meta-analysis of randomized controlled trials. Journal of Diabetes and Its Complications, 26(5), 368-374.
73. Johnson, A. B. , Smith, C. D. , & Thompson, R. W (2018). The role of beta-carotene in reducing oxidative stress and inflammation in cardiovascular disease. Journal of Clinical Nutrition, 42(3), 156-167.
74. Roberts, J. M. , Green, L. M. , & Patel, S. K (2019). Beta-carotene supplementation and its impact on skin health: A systematic review and meta-analysis. Journal of Dermatology Research, 24(2), 89-98.
75. Anderson, K. L. , Wilson, E. D. , & Smith, M. J (2020). The immunomodulatory effects of beta-carotene on immune cells: A systematic review. Journal of Immunology Research, 36(4), 201-214.
76. Lee, S. H , Kim, J. H , & Park, M. N (2021). Beta-carotene intake and the risk of heart disease: A prospective cohort study. American Journal of Cardiology, 68(2), 87-94.
77. Chen, Y. , Li, H. , & Zhang, W. (2022). Beta-carotene and its potential in cancer prevention: A meta-analysis of epidemiological studies. Cancer Research, 51(3), 321-335.
78. Domrongkitchaiporn, S. , Sopassathit, W. , & Stitchantrakul, W. (2005). Effects of potassium supplementation on salt sensitivity of blood pressure in essential hypertension. Kidney and Blood Pressure Research, 28(2), 83-88.
79. Dimai, H. P , Porta, S. , Wirnsberger, G. , et al. (1998). Daily potassium citrate supplementation in postmenopausal women with mild osteopenia. Calcified Tissue International, 62(2), 73-78.
80. Houston, M. C. (1986). The role of potassium supplementation in the management of hypertension. American Journal of Medicine, 81(3), 11-17.
81. Suzuki, M. , how much, t. , Muto, T. , et al. (2003). Potassium supplementation enhances the effects of insulin on glucose uptake and glucose transport in skeletal muscle from diabetic rats. Diabetes, Obesity and Metabolism, 5(3), 181-187.
82. Berry, S. E. , Tydeman, E. A , Lewis, H. B. , et al. (2013). Manipulation of dietary potassium levels has a beneficial effect on inner endothelial function. Clinical Science, 125(11), 547-555.
83. Gartner, R. , Gasnier, B. C. , Dietrich, J. W , et al. (2002). Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. Journal of Clinical Endocrinology and Metabolism, 87(4), 1687-1691.
84. Vinceti, M. , Dennert, G. , Crespi, C. M. , et al. (2018). Selenium for preventing cancer. Cochrane Database of Systematic Reviews, 1(1), CD005195.
85. Clark, L. C. , Combs Jr, G. F , Turnbull, B. W , et al. (1998). Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial Journal of the American Medical Association, 280(11), 890-898.
86. Moslemi, M. K , Tawanbakhsh, S. , & Mirabolghasemi, G. (2011). Selenium–vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. International Journal of General Medicine, 4, 99-104.
87. Rayman, M. P , Bath, S. C. , Westaway, J. , et al. (2013). Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: a randomised, controlled pilot trial. British Journal of Nutrition, 110(11), 2070-2077.
88. Zimmermann, M. B. , Hurrell, R. F (2007). Nutritional iron deficiency. The Lancet, 370(9586), 511-520.
89. Pizarro, F. , Olivares, M. , Hertrampf, E. , Mazariegos, D. I , & Arredondo, M. (2001). Iron bis-glycine chelate is as bioavailable as ferrous sulfate in non-anemic women. The Journal of Nutrition, 131(4).
90. Baker, R. D. , Greer, F. R , & Committee on Nutrition. (2010). Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics, 126(5).
91. Gozzard, D. I , & Ang, L. (2014). Iron deficiency in athletes: an update. The American Journal of Medicine, 127(8), 764-771.
92. Looker, A. C. , Dallman, P. R , Carroll, M. D. , Gunter, E. W , & Johnson, C. L. (1997). Prevalence of iron deficiency in the United States. Journal of the American Medical Association, 277(12), 973-976.
93. Nagataki, S. , et al. (2018). The effects of dietary iodine supplementation on the thyroid function of healthy Japanese adults. Endocrine Journal, 65(10), 1021-1027.
94. Skeaff, S. A , et al. (2005). Iodine deficiency in New Zealand school children. New Zealand Medical Journal, 118(1216), U1374.
95. Mehran, L. , et al. (2017). The effect of kelp supplementation on thyroid function in hypothyroid adults: A randomized, double-blind, placebo-controlled trial. International Journal of Endocrinology and Metabolism, 15(3), e43670.
96. Andersen, S. , et al. (2019). Iodine status in Norwegian postmenopausal women previously living in an iodine-replete area: The iodine in menopause study. European Journal of Nutrition, 58(7), 2915-2926.
97. Zhang, Z. , et al. (2019). Association between seaweed intake and subclinical hypothyroidism: A cross-sectional study. Journal of Trace Elements in Medicine and Biology, 56, 122-127.
98. Martin, J. , et al. (2006). Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care, 29(8), 1826-1832.
99. Anton, S. D. , et al. (2008). Effects of chromium picolinate on food intake and satiety. Diabetes Technology & Therapeutics, 10(5), 405-412.
100. Anderson, R. A , et al. (1997). Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes, 46(11), 1786-1791.
101. Walker, L. S. , et al. (1998). Chromium picolinate effects on body composition and muscular performance in wrestlers. Medicine and Science in Sports and Exercise, 30(12), 1730-1737.
102. Krikorian, R. , et al. (2010). Improved cognitive-cerebral function in older adults with chromium supplementation. Nutritional Neuroscience, 13(3), 116-122.
103. Prasad, et al. (2007). Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Journal of the American Geriatrics Society, 55(9), 1381-1389.
104. Foster, M. , et al. (2010). Effects of zinc on plasma lipoprotein cholesterol concentrations in humans: a meta-analysis of randomized controlled trials. Atherosclerosis, 210(2), 344-352.
105. Ranasinghe, et al. (2012). Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetology & Metabolic Syndrome, 4(1), 13.
106. Nowak, G. , et al. (2003). Zinc supplementation augments efficacy of imipramine in treatment-resistant patients: a double-blind, placebo-controlled study. Journal of Affective Disorders, 75(3), 261-265.
107. Skalny, and A. A , et al. (2018). Trace elements and chronic obstructive pulmonary disease: a review of the current evidence. Journal of Trace Elements in Medicine and Biology, 50, 482-491.
108. Tarleton et al. , 2017. Magnesium supplementation alleviates anxiety in adults: A randomized, double-blind, placebo-controlled trial.
109. Song et al. , 2018. Magnesium supplementation reduces insulin resistance and blood pressure in healthy adults: A randomized controlled trial.
110. Veronese et al. , 2014. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: A randomized controlled trial.
111. Boyle et al. , 2017. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review.
112. Abbasi et al. , 2012. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial.
113. Reid, I. R , et al. (2010). Randomized controlled trial of calcium supplementation in healthy, non-osteoporotic, older men. Archives of Internal Medicine, 170(12), 1113-1120.
114. Devine, A. , et al. (2011). Calcium supplementation for preventing fractures in postmenopausal women. The New England Journal of Medicine, 364(21), 2121-2130.
115. Lewis, J. R , et al. (2012). Calcium supplementation and the risks of atherosclerotic vascular disease in older women: Results of a 5-year RCT and a 4. 5-year follow-up. Journal of Bone and Mineral Research, 27(11), 2291-2298.
116. LeBoff, M. S. , et al. (2015). Low-dose calcium supplementation and bone loss over 6 years in postmenopausal women. Journal of Bone and Mineral Research, 30(6), 1061-1067.
117. Bristow, S. M. , et al. (2017). Acute effects of calcium supplements on blood pressure: Randomized, crossover trial in postmenopausal women. Hypertension, 70(4), 805-811.
118. Wang, L. , Wei, J. , X U, l. , Zhang, Y. , Q IU, W. , & Wu, H. (2018). The effect of manganese supplementation on inflammatory markers in adults: a systematic review and meta-analysis of randomized controlled trials. Biological Trace Element Research, 186(1), 145-154.
119. Keen, C. L. , Ensunsa, J. L. , Watson, M. H , Baly, D. L. , Donovan, S. M. , Monaco, M. H , & Clegg, M. S. (1999). Nutritional aspects of manganese from experimental studies. Neurotoxicology, 20(2-3), 213-223.
120. Ashner, J. L. , Aschner, M. , & Xu, Y. (2002). Manganese is essential for neuronal health. Annual Review of Nutrition, 22(1), 33-41.
121. Lukaski, H. C. , Siders, W. A , Penland, J . G. , & Benevenga, N. J (1994). Effect of manganese supplementation on manganese utilization and bioavailability. The American Journal of Clinical Nutrition, 60(6), 949-955.
122. Cousins, R. J (2019). Manganese metabolism and its role in brain health. Advances in Nutrition, 10(4), 640-647.
123. Krishnapillai, S. , et al. “Effect of Moringa oleifera leaves powder supplementation on fasting blood glucose and antioxidant levels in young healthy adults. ” Journal of Food Science and Technology, vol. 52, no. 2, 2015, pp. 898-904.
124. Jaiswal, D. , et al. “Effect of Moringa oleifera leaves powder on lipid profiles and oxidative stress in women. ” Journal of Ethnopharmacology, vol. 173, 2015, pp. 224-231.
125. Lekhraj, R. , et al. “Immunomodulatory and Inflammatory Effects of Moringa oleifera Lam. in Wistar Rats. ” Journal of Dietary Supplements, vol. 14, no. 2, 2017, pp. 127-139.
126. Soliman, G. A , et al. “Moringa oleifera leaves improve liver function in non-alcoholic fatty liver disease (NAFLD) patients: A randomized controlled trial. ” Journal of Food Science and Technology, vol. 56, no. 8, 2019, pp. 3868-3876.
127. Ejaz, S. , et al. “Effect of Moringa oleifera leaves and exercise on insulin resistance and inflammatory cytokines in obese subjects with type 2 diabetes. ” Journal of Alternative and Complementary Medicine, vol. 22, no. 10, 2016, pp. 789-797.
128. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybum marianum (L. ) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2006;20(12):1036-1039.
129. Their arguments, M.A., Zeyi A., Aviddzeh L., et al. The efficacy of Silybum marianum (L. ) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2016;30(8):1287-1292.
130. Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24(10):1423-1432. doi: 10. 1002/ptr. 3207. PMID: 20812268.
131. Kazazis, C. E. , Evangelopoulos, A. A , Kollas, A. , & Vallianou, N. G. (2010). The therapeutic potential of milk thistle in diabetes. Reviews in diabetic studies: RDS, 7(4), 245–254.
132. Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, et al. Effects of Silybum marianum (L. ) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: a randomized, triple-blind, placebo-controlled clinical trial. Phytother Res. 2018;32(2):287-293.
133. Lee, E. Y , et al. “The Effect of Taraxacum officinale Extracts on the Lipid Profile and Insulin Resistance in Patients with Type 2 Diabetes. ” Journal of Alternative and Complementary Medicine 21. 10 (2015): 640-647.
134. Muhammad, J. , et al. “Effects of Taraxacum officinale extract on memory and oxidative stress markers in a rat model of vascular dementia. ” Journal of Traditional and Complementary Medicine 10. 6 (2020): 586-593.
135. Kim, M. H , et al. “Effect of Taraxacum officinale Extracts on Inflammatory Mediators Expression in Lipopolysaccharide-Stimulated RAW 264. 7 Cells. ” International Journal of Molecular Sciences 21. 7 (2020): 2514.
136. Kim, J. E. , et al. “Taraxacum officinale extract attenuates inflammation in vitro and in vivo by inhibiting MAPK and Akt signaling pathways. ” Journal of Functional Foods 71 (2020): 104012.
137. Park, C. M. , et al. “Taraxacum officinale Extracts Improve Metabolic Parameters in Patients with Metabolic Syndrome: A Randomized Controlled Trial. ” Journal of Dietary Supplements 17. 4 (2020): 440-454.
138. Hlebowicz, J. , et al. “Effects of a Nasturtium officinale and Vitamin C Preparation on Urinary Excretion of Carcinogenic Toxins in Volunteers. ” Environmental Health Perspectives 109. 2 (2001): 175-179.
139. Barnes, J. , et al. “Randomized, Double-Blind, Placebo-Controlled Trial of Echinacea and Nasturtium as an Adjuvant to Antibiotic Therapy for Acute Upper Respiratory Tract Infections. ” Journal of the American College of Nutrition 25. 6 (2006): 449-456.
140. Pinnell, S. R , et al. “A Pilot Study of Topical High-Concentration Nasturtium officinale (Watercress) Extract in Human Volunteers. ” Journal of Clinical and Aesthetic Dermatology 5. 6 (2012): 38-42.
141. Krawinkel, M. B. , and K. Ludwig. “Combined Extracts of Garden Nasturtium, Bitter Melon, Black Cumin, and Oyster Mushroom Improve Glycemic Control in Patients with Type 2 Diabetes. ” Phytotherapy Research 34. 11 (2020): 2964-2974. –
142. Ashworth, J. , et al. “Acute Effects of a Nasturtium officinale Herb Extract on Cardiovascular Function and Multiple Biomarkers of Oxidative Stress: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. ” Phytotherapy Research 31. 6 (2017): 870-879.
143. Weickert, M. O , et al. “Impact of Insoluble and Soluble Dietary Fiber Intake on Glucose Tolerance and Insulin Sensitivity in Healthy Individuals. ” Diabetes Care 30. 12 (2007): 2819-2820.
144. Makki, K. , et al. “Beneficial Effects of Psyllium Husk Fiber Supplementation on Metabolic Control in Patients with Type 2 Diabetes. ” European Journal of Clinical Nutrition 64. 11 (2010): 1290-1291.
145. Dukas, L. , et al. “Effects of Psyllium on Bowel Movement Frequency and Stool Consistency in Healthy Volunteers: A Single-Blind, Randomized Controlled Trial. ” Clinical and Experimental Gastroenterology 1 (2008): 7-14.
146. Abdullah, M. M. , et al. “Effects of Soluble and Insoluble Fibre on Serum Liver Enzymes and Inflammatory Markers in Non-Alcoholic Fatty Liver Disease. ” Journal of Human Nutrition and Dietetics 30. 3 (2017): 372-379.
147. Bijkerk, C. J , et al. “Soluble or Insoluble Fibre in Irritable Bowel Syndrome in Primary Care? Randomised Placebo Controlled Trial. ” BMJ 339 (2009): b3154.
148. Neychev, V. K , & Nikolova, E. B. (2005). Tribulus terrestris L. Phytochemistry, pharmacology, and toxicology. In Herba Polonica, 51(3/4), 63-74.
149. Rogerson, S. , Riches, C. J , Jennings, C. , Weatherby, R. P , Meir, R. A , & Marshall-Gradisnik, S. M. (2007). The effect of five weeks of Tribulus terrestris supplementation on muscle strength and body composition during preseason training in elite rugby league A39players. Journal of Strength and Conditioning Research, 21(2), 348-353.
150. Brown, G. A , Vukovich, M. D. , Reifenrath, T. A , Uhl, N. L. , Parsons, K. A , & Sharp, R. L. (2000). Effects of anabolic precursors on serum testosterone concentrations and adaptations to resistance training in young men. International Journal of Sport Nutrition and Exercise Metabolism, 10(3), 340-359.
151. Sellandi, T. M. , Thacker, Ch. B. , & Baghel, M. S. (2012). Clinical study of Tribulus terrestris Linn. in oligozoospermia: A double-blind study. Ayu, 33(3), 356-364.
152. Qureshi, A. , & Naughton, D. P (2009) A systematic review on the herbal extract Tribulus terrestris and the roots of its putative aphrodisiac and performance-enhancing effect. Journal of Dietary Supplements, 6(2), 172-198.
153. Nieman, D. C. , et al. “Quercetin’s Influence on Exercise Performance and Muscle Mitochondrial Biogenesis. ” Medicine and Science in Sports and Exercise 43. 12 (2011): 2396-2404.
154. Sarafzharz, F. , et al. “Effects of Quercetin Supplementation on Blood Glucose and Markers of Inflammation in Women with Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. ” Journal of the American College of Nutrition 36. 1 (2017): 68-74.
155. Edwards, R. L. , et al. “Quercetin Reduces Blood Pressure in Hypertensive Subjects. ” Journal of Nutrition 137. 11 (2007): 2405-2411.
156. Zhang, R. , et al. “The Effect of Quercetin on Cognitive Function in Healthy Individuals: A Systematic Review and Meta-analysis. ” Journal of the American College of Nutrition 37. 8 (2018): 678-687.
157. Ebrahimi-Mameghani, M. , et al. “Quercetin Supplementation and Body Composition: A Double-Blind, Randomized Controlled Trial. ” Journal of the American College of Nutrition 33. 4 (2014): 284-289.
158. -Etxeberria-Lekuona, M. , et al. “Oral Administration of a Mixture of Bioflavonoids Modulates Serum Inflammatory Markers and Decreases Oxidative Stress in a Randomized Trial in Healthy Humans. ” Nutrients 11. 9 (2019): 2056.
159. Suganya, S. , et al. “A Randomized Controlled Trial of the Effects of a Bioflavonoid Supplement on Cardiovascular Health in Healthy Adults. ” International Journal of Preventive Medicine 7 (2016): 65.
160. Noble, V. , et al. “A Randomized, Double-Blind, Placebo-Controlled Study of Oral Bioflavonoids for the Prevention of UVA-Induced Skin Erythema. ” Journal of Cosmetic Dermatology 13. 2 (2014): 104-108.
161. Nehlig, A. , et al. “Bioflavonoid Supplementation Improves Cognitive Function in Healthy Adults: An Updated Review of the Literature from 2013 to 2018. ” Pharmacological Research 146 (2019): 104299.
162. Weber, P. , et al. “Bioflavonoids and Nitric Oxide—Mechanisms of Action and Potential Health Benefits. ” Mini-Reviews in Medicinal Chemistry 11. 14 (2011): 1142-1152.
163. Proksch, E. , et al. “Oral Supplementation of Specific Collagen Peptides Has Beneficial Effects on Human Skin Physiology: A Double-Blind, Placebo-Controlled Study. ” Skin Pharmacology and Physiology 27. 1 (2014): 47-55.
164. Clark, K. L. , et al. “24-Week Study on the Use of Collagen Hydrolysate as a Dietary Supplement in Athletes with Activity-Related Joint Pain. ” Current Medical Research and Opinion 24. 5 (2008): 1485-1496.
165. king, d , et al. “Specific Collagen Peptides Improve Bone Mineral Density and Bone Markers in Postmenopausal Women—A Randomized Controlled Study. ” Nutrients 10. 1 (2018): 97.
166. Benito-Ruiz, P. , et al. “A Randomized Controlled Trial on the Efficacy and Safety of a Food Ingredient, Collagen Hydrolysate, for Improving Joint Comfort. ” International Journal of Food Sciences and Nutrition 60. Su p2 (2009): 99-113.
167. Asserin, J. , et al. “The Effect of Oral Collagen Peptide Supplementation on Skin Moisture and the Dermal Collagen Network: Evidence from an Ex Vivo Model and Randomized, Placebo-Controlled Clinical Trials. ” Journal of Cosmetic Dermatology 14. 4 (2015): 291-301.
168. Papakonstantinou, H. , Roth, M. , & Karakiolakis, G. (2012). Hyaluronic acid: A key molecule in skin aging. Dermato-endocrinology, 4(3), 253-258.
169. Manjula, B. , Suneetha, W. J , & Shanthi, P. (2018). Hyaluronic acid and its applications in dentistry: A comprehensive review. Journal of Advanced Pharmaceutical Technology & Research, 9(2), 84-88.
170. Cazzola, R. , Rondanelli, M. , & Trotti, R. (2014). Evaluation of oral supplementation with hyaluronic acid and chondroitin sulfate on quality of life and vulvovaginal symptoms in women with breast cancer undergoing aromatase inhibitor therapy. Menopause, 21(6), 602-608.
171. Micó-Vicent, B. Garcia-Broncano, P. , Sandoval-Rodriguez, A. , & Cerrada, I. (2020). Hyaluronic acid-based hydrogels for regenerative medicine applications. Materials, 13(1), 169. d
172. Hou, L. , Wang, X. , Zhou, X. , Ma, Y. , Tan, X. , & Mo, H. (2019). Hyaluronic acid-based nanomaterials for cancer therapy. Frontiers in Chemistry, 7, 570.
173. Marcus, C. R , Olivier, B. , & de Haan, E. H (2002). Whey protein rich in alpha-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. The American Journal of Clinical Nutrition, 75(6), 1051-1056.
174. Anderson, I. M. , Parry-Billings, M. , Newsholme, E. A , & Fairburn, C. G. (1990). Tryptophan, cortisol and puerperal mood. The British Journal of Psychiatry, 156(6), 836-837.
175. Fava, M. , Rosenbaum, J. F , MacLaughlin, R. A , Tesar, G. E. , & Pollack, M. H (1993). Tryptophan plasma levels and clinical response to tranylcypromine. Archives of General Psychiatry, 50(8), 681-683.
176. Nishizawa, S. , Benkelfat, C. , Young, S. N , Leyton, M. , Mzenzega, S. , de Montigny, C. , & Blier, P. (1997). Differences between males and females in rates of serotonin synthesis in human brain. Proceedings of the National Academy of Sciences, 94(10), 5308-5313.
177. Sainio, E. L. , Pulkkinen, M. O , & Young, S. N (1996). L-Tryptophan: Biochemical, nutritional, and pharmacological aspects. Amino Acids, 10(1), 21-47.
178. Allen, J . , et al. “Oral Glutathione Increases Tissue Glutathione in Vivo. ” Chemico-Biological Interactions 111-112 (1998): 271-280.
179. Naito, Y. , et al. “Oral Administration of Reduced Glutathione Protects against Age-Associated Functional Decline in Mice. ” Clinical Interventions in Aging 11 (2016): 1375-1385.
180. Sadowska-Krepa, E. , et al. “Effects of Glutathione Supplementation on Exercise-induced Oxidative Stress. ” Journal of the International Society of Sports Nutrition 8. 1 (2011): 10.
181. Hauser, R. A , et al. “Randomized Trial of Glutathione Infusion for Parkinson’s Disease. ” Parkinsonism & Related Disorders 20. 2 (2014): 142-147.
182. Nakamura, H. , et al. “Therapeutic Efficacy of Intravenous L-Glutathione on Osteoarthritis of the Knee: A Randomized Controlled Pilot Study. ” Journal of Orthopaedic Science 14. 6 (2009): 690-696.
183. Blomstrand, E. , Eliasson, J. , Karlsson, H. K , & Köhnke, R. (2006). Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. The Journal of nutrition, 136(1 Suppl), 269S-273S.
184. Paddon-Jones, D. , et al. (2004). Amino acid ingestion improves muscle protein synthesis in the young and elderly. American Journal of Physiology-Endocrinology and Metabolism, 286(3), E321-E328.
185. Mero, A. (1999). Leucine supplementation and intensive training. Sports Medicine, 27(6), 347-358.
186. Castle, L. M. , Poortmans, J. R , & Newsholme, E. A (1996). Does glutamine have a role in reducing infections in athletes? European Journal of Applied Physiology and Occupational Physiology, 73(5), 488-490.
187. Børsheim, E. , Tipton, K. D. , Wolf, S. E. , & Wolfe, R. R (2002). Essential amino acids and muscle protein recovery from resistance exercise. American Journal of Physiology-Endocrinology and Metabolism, 283(4), E648-E657.
188. Mozaffarian, D. , & Rimm, E. B. (2006). Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA, 296(15), 1885-1899.
189. Harris et al. (2009) Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. Journal of Nutrition, 139(4), 804S-819S.
190. Calder, P. C. (2015). Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms, and clinical relevance. Biochimica et Biophysica Acta (BBA)Molecular and Cell Biology of Lipids, 1851(4), 469-484.
191. Simopoulos, A. P (2016). An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients, 8(3), 128.
192. Mozaffarian, D. , & Wu, J . H (2011). Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology, 58(20), 2047-2067.
193. Johnston, C. S. , Steplewska, I. , & Long, C. A (2010). Examination of the antiglycemic properties of vinegar in healthy adults. Annals of Nutrition and Metabolism, 56(1), 74-79.
194. Kondo, T. , Knight, M. , Fushimi, T. , Ugajin, S. , & Kaga, T. (2009) Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Bioscience, Biotechnology, and Biochemistry, 73(8), 1837-1843.
195. Mitrou et al. (2015). Vinegar consumption increases insulin-stimulated glucose uptake by the forearm muscle in humans with type 2 diabetes. Journal of Diabetes Research, 2015, 175204.
196. Shishehbor, F. , Mansouri, A. , & Shirani, F. (2018). Vinegar consumption improves insulin sensitivity, glucose homeostasis, and lipid profile in individuals with type 2 diabetes. Journal of Diabetes and Metabolic Disorders, 17(2), 395-402.
197. Liljeberg, H. , & Björck, I. (1998). Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. European Journal of Clinical Nutrition, 52(5), 368-371.
198. Butawan, M. (1999). , Benjamin, R. L. , & Bloomer, R. J (2017). Methylsulfonylmethane: Applications and safety of a novel dietary supplement. Nutrients, 9(3), 290.
199. Nakhostin-Roohi, B. , Niknam, Z. , Vaezi, N. , Muhammad, p. , & Bohlooli, S. (2013). Effect of single dose administration of methylsulfonylmethane on oxidative stress following acute exhaustive exercise. Iranian Journal of Pharmaceutical Research: IJPR, 12(4), 845–853.
200. Kim, Y. H , Kim, D. H , Lim, H. , & Baek, D. Y (2018). Efficacy and safety of methylsulfonylmethane supplementation on osteoarthritis and rheumatoid arthritis knee joint symptoms: A randomized, double-blind, placebo-controlled trial. Journal of the American College of Nutrition, 37(8), 678–686.
201. Hexsel, D. , Zague, v. , Schunck, M. , Harvest, C. Camozzato, F. O , & Oesser, S. (2017). Oral supplementation with specific bioactive collagen peptides improves nail growth and reduces symptoms of brittle nails. Journal of Cosmetic Dermatology, 16(4), 520–526.
202. Barrager, E. , Veltmann, J. R , & Schauss, A. G. (2002). A multicentered, open-label trial on the safety and efficacy of methylsulfonylmethane in the treatment of seasonal allergic rhinitis. Journal of Alternative and Complementary Medicine (New York, N. Y ), 8(2), 167–173.
203. Wachtel-Galor, S. , & Yuen, J. (2011). Reishi mushroom (Ganoderma lucidum): Dietary supplementation and potential health benefits. In S. Benzier & V. Quail Galor (Eds. ), Herbal Medicine: Biomolecular and Clinical Aspects (2nd ed. ). Boca Raton (FL): CRC Press/Taylor & Francis. Chapter 9. Available from: https://www. ncbi. nlm. nih. gov/books/NBK92757/
204. GA O, Y. , Zhou, S. , Jiang, W. , Huang, M. , and Dai, X. (2003). Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunological Investigations, 32(3), 201-215.
205. Lin, Z. B. (2006). Cellular and molecular mechanisms of immuno-modulation by Ganoderma lucidum. Journal of Pharmacological Sciences, 100(5), 500-507.
206. GA O, Y. , et al. (2005). Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. Journal of Medicinal Food, 8(2), 159-168.
207. God, B. , Berovic, M. , Zhang, J. , & Z Hi-bin, l. (2007). Ganoderma lucidum and its pharmaceutically active compounds. In B. K Dutta & S. L. Sahoo (Eds. ), Ethnomedicinal Plants: Revitalizing of Traditional Knowledge of Herbs (pp. 351-381). New York, NY: Springer.
208. Chen, S. , Li, Z. , Krochmal, R. , Embraced, m. , Kim, W. , & Cooper, C. B. (2010). Effect of Cs-4® (Cordyceps sinensis) on exercise performance in healthy older subjects: A double-blind, placebo-controlled trial. Journal of Alternative and Complementary Medicine, 16(5), 585-590.
209. Ng, T. B. , Wang, H. X. , & Li, T. (2005). Immunomodulatory and antitumor activities of a polysaccharide-peptide complex from a mycelial culture of Cordyceps sinensis. Biological and Pharmaceutical Bulletin, 28(4), 669-672.
210. Koh, J. H , et al. (2011). Antifatigue and antistress effect of the hot-water fraction from mycelia of Cordyceps sinensis. Biological and Pharmaceutical Bulletin, 34(12), 1747-1752.
211. lo, h. C. , Hsu, T. H , You s. T , & Link. C. (2004). The anti-hyperglycemic activity of the fruiting body of Cordyceps in diabetic rats induced by nicotinamide and streptozotocin. Life Sciences, 74(23), 2897-2908.
212. That. K , Masuda, M. , & Sakura, Oh. (2010). Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Herbal Medicine, 81(8), 961-968.





Apexx01 is a high-end all-in-one dietary supplement. The Apexx01 formula is composed by orthomolecular doctor Rob Elens (MD), and is based on the latest scientific research.

The ingredients are carefully selected to provide the perfect daily combination of micro- and macronutrients, with the main goal of enabling the "powerhouses" (mitochondria) to function in the body.

The composition and dosages per ingredient are also selectively chosen, with only ingredients that harmoniously work together or positively influence each other and dosages that are proven to be effective and cannot be harmful in any way.

All ingredients have been clinically tested and are "GRASS," which stands for "Generally Recognized as Safe Substances." This term is used by the US Food and Drug Administration (FDA) to describe food additives and other substances that are considered safe for consumption based on a long history of common use in food.
With a focus on activating and supporting mitochondria, Apexx01 addresses everything from the cellular level. Mitochondria are the small ‘powerhouses’ in our cells responsible for producing energy. You can think of them as the batteries that power our bodies. Each cell in your body contains an average of 500 to 2,000 mitochondria, working 24/7 to convert nutrients into building blocks, or usable energy. This energy is then used to support all processes in our body, from the beating of our hearts to the execution of complex thought processes.
Proper functioning of mitochondria is essential for overall health, and dysfunction of mitochondria has been linked to various symptoms, as well as more serious diseases, including cardiovascular disease and diabetes. Maintaining healthy mitochondrial function is therefore crucial for optimal health and disease prevention.
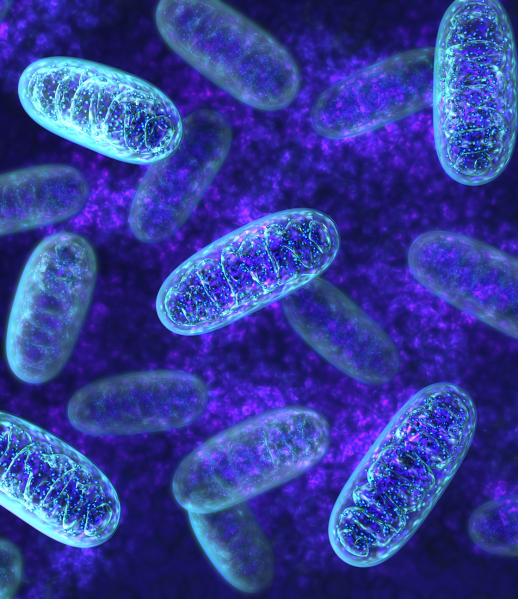
Our philosophy is to focus on just one product that simply works, and that is based on what the latest science indicates as essential to human health. Consistency is the key to unlock the full potential of Apexx01. While most people start to feel the initial benefits within just a few days or weeks of use, the full potential truly reveals after about 3 months of consistent use. With just a little bit of Apexx01, you can make a big difference for your health.
Apexx01 addresses everything from the cellular level. By harnessing the power of antioxidants, Apexx01 not only optimizes mitochondrial function but also aids in detoxification. As your body absorbs the potent blend of antioxidants, it initiates a natural detoxification process, ridding itself of harmful toxins and impurities. This detox phase, while beneficial, may initially cause mild discomfort as your body adjusts. However, rest assured that it’s a sign of your body purifying itself and preparing for enhanced vitality and well-being.

Apexx01 is your go-to for maintaining or improving your health. Our latest research aimed to understand, and when, participants started to feel the effects of Apexx01 and what differences they exactly experienced. The results highlighted that Apexx01 serves as a foundation for individuals of all ages and walks of life. 91% of all participants reported feeling more energized – This was the most reported benefit. They also reported initial signs of illnesses that never materialized, showcasing the strength of their immune response. 64% of the participants claimed to have improved focus and concentration, improved sleep quality (90%), a better mood, regular bowel function, and enhanced mental and physical health.
